|
|
|
|
IRBNet operates the most widely used solution for managing research compliance. The National Research Network serves more than 1600 organizations across all 50 states.
|
|








Download the National Research Network 2011 Benchmark Report




For a complete copy of the
National Research Network 2011 Benchmark Report click here. 
To learn how your institution can use IRBNet's powerful data and metrics to assess and improve your HRPP operations and performance, visit us at www.irbnet.org.
If you would like to automatically receive future copies of National Benchmark Reports send an email to benchmark@irbnet.org.
|

|





How Do You Compare?


The National Research Network ® 2011 Benchmark Report includes data from more than 69 randomly selected institutions active on the National Research Network, sampled from April 2010 - March 2011. Anonymous selection was based upon tenure, volume of activity, institutional profile, completeness of data and other factors.
The 2011 Benchmark Report pulls together real world performance data from hospitals, universities and government agencies across the country. It is provided as a service to the IRB community to assist organizations as they benchmark internal performance. More than 90 separate metrics are presented for biomedical focused and social / behavioral-focused research institutions.
|
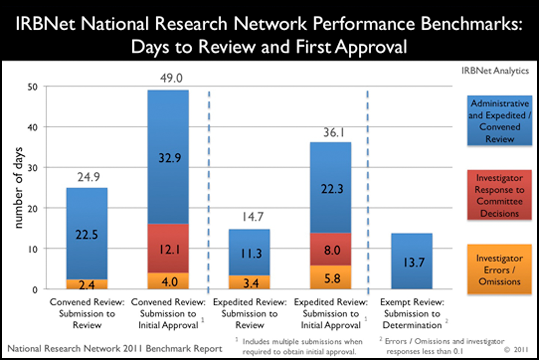
Highlight:
32% of time to first approval by convened review is due to investigator errors and required rework.
|


|
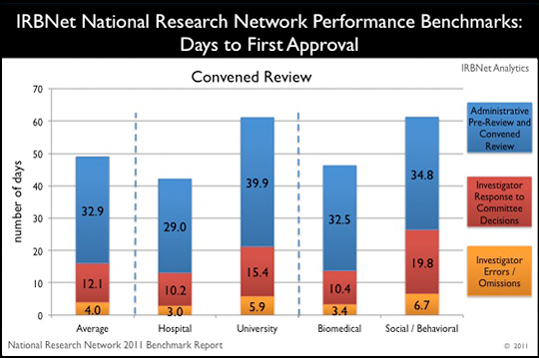
Highlight:
Hospitals approve new studies via convened review faster than the national average.
|


|
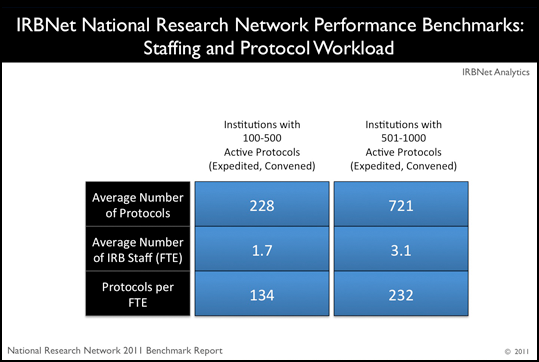
Highlight:
National Research Network members efficiently manage large protocol workloads with an average of only 3 Staff full time equivalents (FTEs).
|


|






For a complete copy of the National Research Network ® 2011 Benchmark Report click here. 
If you would like to automatically receive future copies of National Benchmark Reports send an email to benchmark@irbnet.org.
top







Why IRBNet? Three Keys to Success.
1. The Complete Enterprise Solution
IRBNet is the leading solution for managing research compliance and oversight, seamlessly powering IRB, IACUC, Biosafety, COI, Stem Cell, Sponsored Programs, Publication Clearance, Training and Credentials Management and other important oversight functions on a unified platform. Members benefit from participation in the largest research and compliance oversight network in the country.
IRBNet members include hospitals and hospital networks, universities and research institutions, and federal and state agencies.
2. Flexible, Intuitive, Easy to Use
Your own forms, processes, and standards. Powerful reporting. The performance metrics and data you need. Electronic submissions. Form wizards. Agendas, minutes, correspondence and more.
Easy to use, web-based tools that are rapidly launched and backed by best practices expertise and the industry's leading support team.
3. Secure, Reliable, Cost-Effective
IRBNet's secure web-based solution is accessible to your research community anytime, anywhere. Our enterprise-class technology serves institutions and health networks of every size.
IRBNet National Research Network Members benefit from streamlined processes, reduced information technology and operational costs, shortened cycle times, greater process transparency and superior data reporting.
top
|
2012 Events
PRIM&R Annual IACUC Conference
Boston MA, March 20–21
OHRP Regional Forum
Raleigh NC, March 21-22
AAHRPP Annual Conference
Denver CO, April 18-20
Michigan Research Ethics Conference
Grand Rapids, MI, April 27
NAIM Annual Conference
Atlanta GA, May 10-11
HCCA Research Compliance Conference
Austin TX, June 3-6
NCURA 54th Annual Meeting
Washington DC, November 4–7
PRIM&R 2012 Advancing Ethical Research Conference
San Diego CA, December 4-6
|
Online Demo

Editor's note
This newsletter is meant to inform and provoke thought on key issues in research compliance. We will continue to probe issues of importance in future newsletters. Please do not hesitate to write with suggestions for future topics or individuals to interview.
If you know someone who may be interested in receiving this newsletter, you can easily
forward up to five copies
at once.
Andrew Olmsted
IRBNet
PO Box 1325
Lebanon, NH 03766
www.irbnet.org
news@irbnet.org |
|